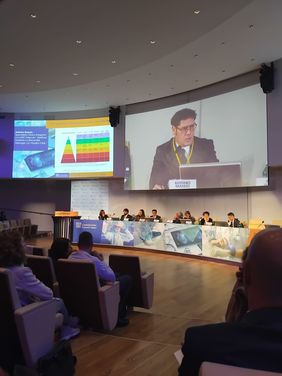
Yesterday (10/16/2024), MTIC InterCert, participated in the Regulatory Affairs Day (#RADay2024) in Rome, organized by Confindustria Medical Devices. This important event brought together industry leaders and regulatory experts to discuss the latest updates on European regulations on medical devices (MDR) and in vitro diagnostics (IVDR).
Dr. Antonio Brando, Integrated Clinical Specialist at MTIC InterCert, was one of the speakers, covering the topic, “Clinical data: how to make the most of its collection for conformity assessment?”
As a Notified Body (CE 0068), MTIC InterCert is proud to support industry with rigorous certification processes, ensuring compliance and safety in line with MDR.